A historical reference laboratory focused on the future
Since its creation in 1967, Laboratoire Cerba operates on the French and international market of specialized clinical pathology.
As a European leader, Laboratoire Cerba aims at supporting health professional by offering them an always up-to-date portfolio of in vitro diagnostic tests and by permanently improving its service.
Highly diversified activity of Laboratoire Cerba covers all medical specialties. It is organized around 3 departments, namely Clinical Pathology, Genetics and Histocytopathology.
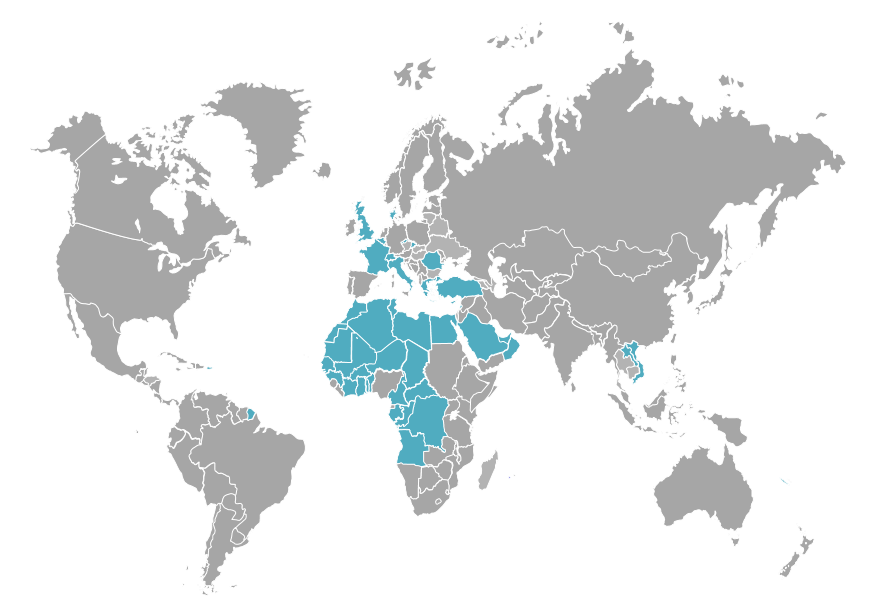
A panel of over 2000 tests and continuous development of new biomarkers are the core of the health care extension provided to clinical pathologists working with Laboratoire Cerba, wherever in the world. This is why Laboratoire Cerba processes samples from all over the world and collects them directly in over 16 countries in Europe, Africa and Middle East through a network of agencies sharing its values and requirements in terms of quality and medical service provided.
In 2007, Cerba was at the origin of the creation of Cerba HealthCare, a European network of laboratories providing a comprehensive solution for clinical pathology, from collection centers to specialized laboratory. Cerba HealthCare also operates worldwide in the field of clinical pathology for clinical trials.
Tests
Patient files/day
Customers worldwide
Laboratoire Cerba’s Medical Team is made of professionals with skills acknowledged by their peers. Members of scientific societies, experts for health authorities, all clinical pathologists regularly attend national and international congresses in their field. They support and advise health professionals in choosing the most relevant tests and in interpreting the results to improve patient’s management.
Laboratoire Cerba integrates a Scientific and Medical board to empower the scientific and technologic survey for continuous evolution of analytical methods and transfer of latest outputs of research to biomedical applications. This board organizes the synergies between our multidisciplinary teams who attend several conferences and regularly publish in national and international medical journals.
Physicians and clinical pathologists
Laboratory technicians
Medical specialties
Laboratoire Cerba was the first laboratory to be accredited by the COFRAC in France as early as April 1999. This long experience of accreditation is a strong driving force for all men and women working at Laboratoire Cerba.
Quality management at Laboratoire Cerba aims at ensuring, for all customers worldwide :
Since 2007, Laboratoire CERBA keeps its position of European leader on the market of 2nd intention clinical pathology and becomes the specialized clinical pathology department of the French laboratory network of Cerba HealthCare group.
The Medical & Scientific board of Laboratoire Cerba aims at defining major developement axis for new diagnostic tools to be proposed to physicians and patients.
Therefore, it maintains a constant scientific survey :
In addition, it leads the medical and scientific teams of Laboratoire Cerba using their multidisciplinary characteristics to implement and develop all synergies for our laboratory to position itself as a major actor in the clinical diagnostic process.
Aurélie Driss-Crorbin
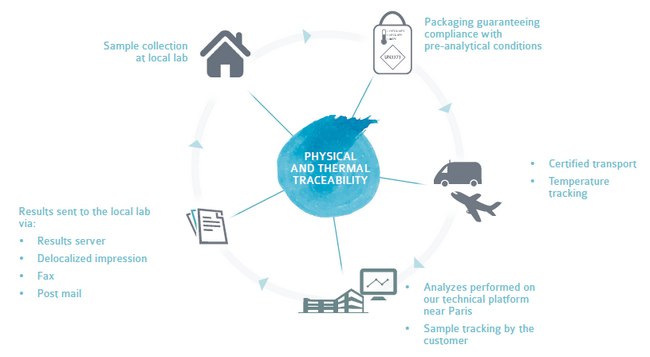
Laboratoire Cerba offers full support for your samples ensuring :
With over 560 collaborators and 120 M€ TO, Laboratoire CERBA is the European leader in specialized clinical pathology.
The values of Laboratoire Cerba are based on :
Those values illustrate the positioning of Laboratoire Cerba in a modern and human perspective of clinical pathology. These values illustrate the will to position in a modern and human perspective of the medical biology. It forms a positive step towards developing a future-oriented clinical pathology.
Human values embedded in this positioning are:
« The values of men fitting with the values of the laboratory »
In agreement with this belief, the laboratory has signed since 2006 the French Convention for Diversity. Diversity is put at the heart of its Human Resources management policy as a factor of development and achievement.
Our laboratory is also authorized for the following tests :
Prenatal diagnosis operations
Cytogenetic tests
Molecular genetic tests
Tests for diagnostic purposes of infectious diseases
Biochemistry tests, maternal markers
Postnatal diagnosis operations
Cytogenetic tests, including molecular cytogenetics
Molecular genetic tests
Lead measurement
Other authorizations
Radio-immunology n°I.S. 214L3C